Implant Syringe
ready-to-fill |
ready-to-sterilize
Drug Device System
is used for the insertion of long-acting (retarding), bio-degradable medicines.
From dossier preparation for the implant to filling: Leveraging our partner network, we offer you a comprehensive support.
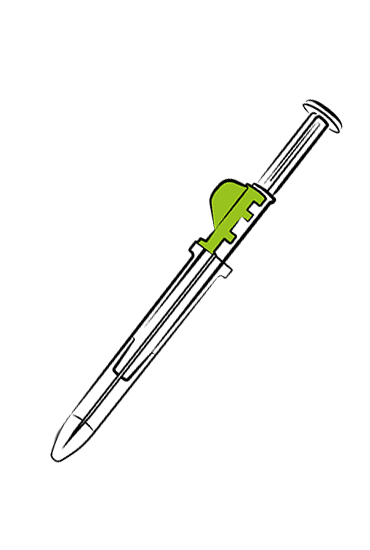
These implants are primarily used for hormone therapies in the following areas:

Application

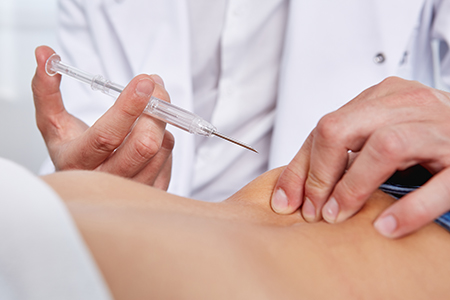
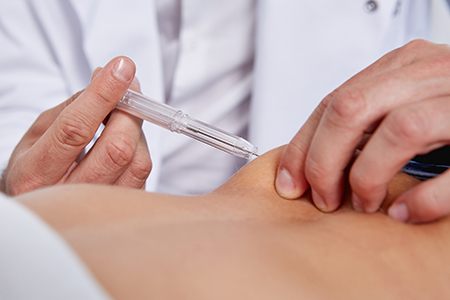
After removing the safety clip and protective cap, the doctor positions the implant syringe and performs a subcutaneous injection into the puncture channel. Depressing the plunger fully, the implant is delivered into the needle channel initially, and then the needle automatically retracts from the tissue into the integrated needle guard. This ensures prevention of needle-related injuries and minimizes infection risks. Finally, the syringe can be disposed of properly.
Do you have any questions about possible custom modifications or production standards?
At the customer’s or white-label producer’s facility, the implant is inserted from the rear into the syringe body (BG1) or into the depot, and then finally assembled with the plunger (BG2).
The implant syringe can be sterilized as follows: